
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Guideline/Fact Sheet
- Dyslipidemia Fact Sheet in South Korea, 2022
- Eun-Sun Jin, Jee-Seon Shim, Sung Eun Kim, Jae Hyun Bae, Shinae Kang, Jong Chul Won, Min-Jeong Shin, Heung Yong Jin, Jenny Moon, Hokyou Lee, Hyeon Chang Kim, In-Kyung Jeong, on Behalf of the Committee of Public Relation of the Korean Society of Lipid and Atherosclerosis
- Diabetes Metab J. 2023;47(5):632-642. Published online August 2, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0135
- 3,047 View
- 315 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
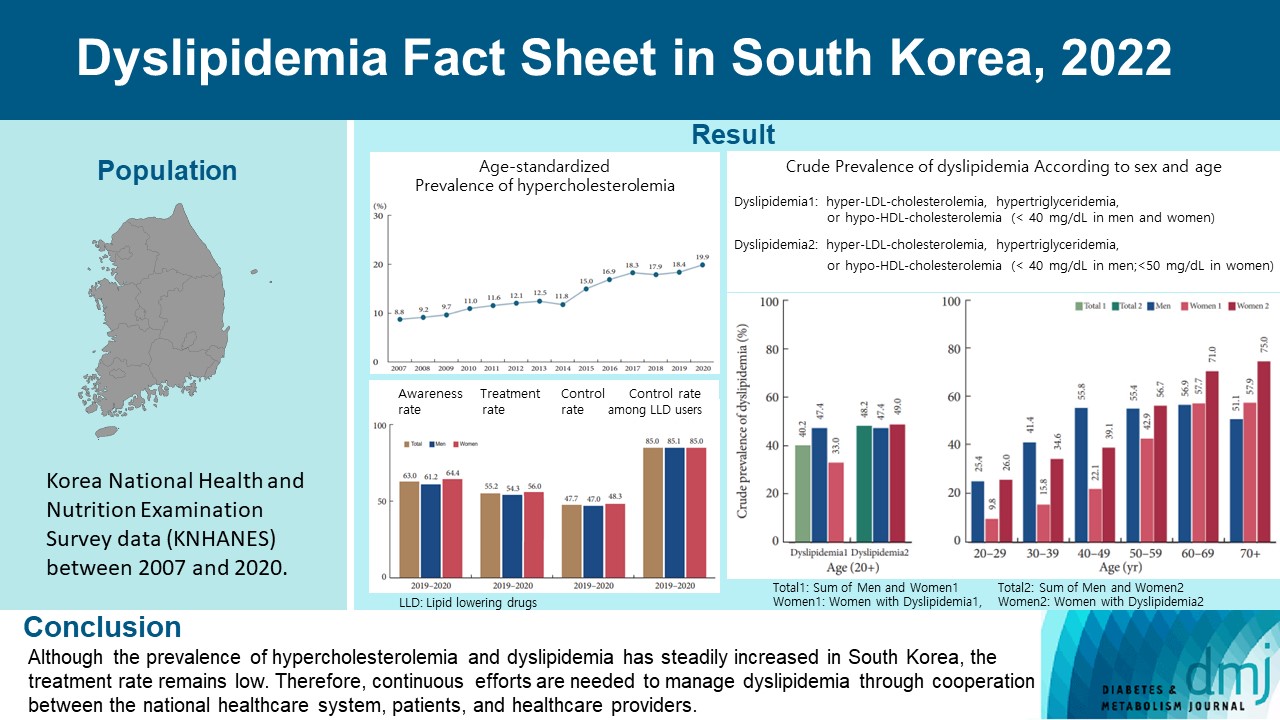
This study aimed to investigate the prevalence and status of dyslipidemia management among South Korean adults, as performed by the Korean Society of Lipid and Atherosclerosis under the name Dyslipidemia Fact Sheet 2022.
Methods
We analyzed the lipid profiles, age-standardized and crude prevalence, management status of hypercholesterolemia and dyslipidemia, and health behaviors among Korean adults aged ≥20 years, using the Korea National Health and Nutrition Examination Survey data between 2007 and 2020.
Results
In South Korea, the crude prevalence of hypercholesterolemia (total cholesterol ≥240 mg/dL or use of a lipid-lowering drug) in 2020 was 24%, and the age-standardized prevalence of hypercholesterolemia more than doubled from 2007 to 2020. The crude treatment rate was 55.2%, and the control rate was 47.7%. The crude prevalence of dyslipidemia—more than one out of three conditions (low-density lipoprotein cholesterol ≥160 or the use of a lipid-lowering drug, triglycerides ≥200, or high-density lipoprotein cholesterol [HDL-C] [men and women] <40 mg/dL)—was 40.2% between 2016 and 2020. However, it increased to 48.2% when the definition of hypo-HDL-cholesterolemia in women changed from <40 to <50 mg/dL.
Conclusion
Although the prevalence of hypercholesterolemia and dyslipidemia has steadily increased in South Korea, the treatment rate remains low. Therefore, continuous efforts are needed to manage dyslipidemia through cooperation between the national healthcare system, patients, and healthcare providers. -
Citations
Citations to this article as recorded by- Oxidative Balance Score and New-Onset Type 2 Diabetes Mellitus in Korean Adults without Non-Alcoholic Fatty Liver Disease: Korean Genome and Epidemiology Study-Health Examinees (KoGES-HEXA) Cohort
Mid-Eum Moon, Dong Hyuk Jung, Seok-Jae Heo, Byoungjin Park, Yong Jae Lee
Antioxidants.2024; 13(1): 107. CrossRef - Comparison of metabolic and neurological comorbidities in Asian patients with psoriasis and atopic dermatitis
Hee Joo Yang, Mi Young Lee, Jeong Hyeon Lee, Chang Jin Jung, Woo Jin Lee, Chong Hyun Won, Mi Woo Lee, Joon Min Jung, Sung Eun Chang
Scientific Reports.2024;[Epub] CrossRef - Effect of Adding Apolipoprotein B Testing on the Prevalence of Dyslipidemia and Risk of Cardiovascular Disease in the Korean Adult Population
Rihwa Choi, Sang Gon Lee, Eun Hee Lee
Metabolites.2024; 14(3): 169. CrossRef - Exploring Utilization and Establishing Reference Intervals for the Apolipoprotein B Test in the Korean Population
Rihwa Choi, Sang Gon Lee, Eun Hee Lee
Diagnostics.2023; 13(20): 3194. CrossRef
- Oxidative Balance Score and New-Onset Type 2 Diabetes Mellitus in Korean Adults without Non-Alcoholic Fatty Liver Disease: Korean Genome and Epidemiology Study-Health Examinees (KoGES-HEXA) Cohort
- Response: Association between Non-Alcoholic Steatohepatitis and Left Ventricular Diastolic Dysfunction in Type 2 Diabetes Mellitus (
Diabetes Metab J 2020;44:267–76) - Hokyou Lee, Gyuri Kim, Yong-ho Lee
- Diabetes Metab J. 2020;44(3):486-487. Published online June 29, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0127
- [Original]
- 3,901 View
- 51 Download
- 1 Crossref
-
 PDF
PDF PubReader
PubReader -
Citations
Citations to this article as recorded by- Association of Metabolic Dysfunction-Associated Fatty Liver Disease With Left Ventricular Diastolic Function and Cardiac Morphology
Dandan Peng, Zhenqiu Yu, Mingwei Wang, Junping Shi, Lei Sun, Yuanyuan Zhang, Wenbin Zhao, Chen Chen, Jiake Tang, Chunyi Wang, Jie Ni, Wen Wen, Jingjie Jiang
Frontiers in Endocrinology.2022;[Epub] CrossRef
- Association of Metabolic Dysfunction-Associated Fatty Liver Disease With Left Ventricular Diastolic Function and Cardiac Morphology
- Complications
- Association of Snoring with Prediabetes and Type 2 Diabetes Mellitus: The Cardiovascular and Metabolic Diseases Etiology Research Center Cohort
- So Mi Jemma Cho, Hokyou Lee, Jee-Seon Shim, Hyeon Chang Kim
- Diabetes Metab J. 2020;44(5):687-698. Published online April 16, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0128

- 5,241 View
- 108 Download
- 7 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub Background Evidence suggests that habitual snoring is an independent risk factor for poor glycemic health. We examined the associations between snoring with prediabetes and diabetes in Korean population.
Methods Self-reported snoring characteristics were collected from 3,948 middle-aged adults without prior cardiovascular diseases. Multivariable linear regression assessed the association of snoring intensity, frequency, disruptiveness, and disrupted breathing with fasting glucose and glycosylated hemoglobin (HbA1c) level. Then, multinomial regression evaluated how increasing snoring symptoms are associated with the risk for prediabetes and diabetes, adjusting for socioeconomic and behavioral risk factors of diabetes, obesity, hypertension, and other sleep variables.
Results Higher snoring intensity and frequency were positively associated with fasting glucose and HbA1c levels. Participants presenting the most severe snoring were at 1.84 times higher risk (95% confidence interval [CI], 1.09 to 2.29) for prediabetes and 2.24 times higher risk (95% CI, 1.84 to 2.95) for diabetes, compared to non-snorers. Such graded association was also observed amongst the most frequent snorers with higher risk for prediabetes (odds ratio [OR], 1.78; 95% CI, 1.29 to 2.22) and diabetes (OR, 2.03; 95% CI, 1.45 to 2.85). Disruptive snoring (OR, 1.60; 95% CI, 1.12 to 2.28) and near-daily disruptive breathing (OR, 2.18; 95% CI, 1.02 to 4.19) were associated with higher odds for diabetes. Such findings remained robust after additional adjustment for sleep duration, excessive daytime sleepiness, unwakefulness, and sleep-deprived driving.
Conclusion Snoring is associated with impaired glucose metabolism even in otherwise metabolically healthy adults. Habitual snorers may require lifestyle modifications and pharmacological treatment to improve glycemic profile.
-
Citations
Citations to this article as recorded by- Does seasonality affect snoring? A study based on international data from the past decade
Ping Wang, Cai Chen, Xingwei Wang, Ningling Zhang, Danyang Lv, Wei Li, Fulai Peng, Xiuli Wang
Sleep and Breathing.2023; 27(4): 1297. CrossRef - Association Between Snoring and Diabetes Among Pre- and Postmenopausal Women
Yun Yuan, Fan Zhang, Jingfu Qiu, Liling Chen, Meng Xiao, Wenge Tang, Qinwen Luo, Xianbin Ding, Xiaojun Tang
International Journal of General Medicine.2022; Volume 15: 2491. CrossRef - Elevated fasting insulin results in snoring: A view emerged from causal evaluation of glycemic traits and snoring
Minhan Yi, Quanming Fei, Kun Liu, Wangcheng Zhao, Ziliang Chen, Yuan Zhang
European Journal of Clinical Investigation.2022;[Epub] CrossRef - Sleeping Duration, Napping and Snoring in Association with Diabetes Control among Patients with Diabetes in Qatar
Hiba Bawadi, Asma Al Sada, Noof Al Mansoori, Sharifa Al Mannai, Aya Hamdan, Zumin Shi, Abdelhamid Kerkadi
International Journal of Environmental Research and Public Health.2021; 18(8): 4017. CrossRef - Changes in creatinine‐to‐cystatin C ratio over 4 years, risk of diabetes, and cardiometabolic control: The China Health and Retirement Longitudinal Study
Shanhu Qiu, Xue Cai, Yang Yuan, Bo Xie, Zilin Sun, Tongzhi Wu
Journal of Diabetes.2021; 13(12): 1025. CrossRef - Association Between Self-Reported Snoring and Metabolic Syndrome: A Systematic Review and Meta-Analysis
Jinsha Ma, Huifang Zhang, Hui Wang, Qian Gao, Heli Sun, Simin He, Lingxian Meng, Tong Wang
Frontiers in Neurology.2020;[Epub] CrossRef - Early Development of Bidirectional Associations between Sleep Disturbance and Diabetes
Yongin Cho
Diabetes & Metabolism Journal.2020; 44(5): 668. CrossRef
- Does seasonality affect snoring? A study based on international data from the past decade
- Metabolic Risk/Epidemiology
- Sex-, Age-, and Metabolic Disorder-Dependent Distributions of Selected Inflammatory Biomarkers among Community-Dwelling Adults
- So Mi Jemma Cho, Hokyou Lee, Jee-Seon Shim, Hyeon Chang Kim
- Diabetes Metab J. 2020;44(5):711-725. Published online April 16, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0119
- 5,917 View
- 83 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
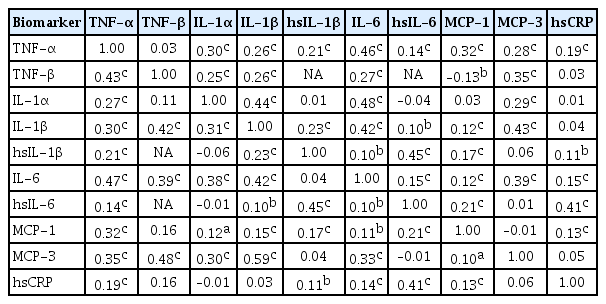
ePub Background Inflammatory cytokines are increasingly utilized to detect high-risk individuals for cardiometabolic diseases. However, with large population and assay methodological heterogeneity, no clear reference currently exists.
Methods Among participants of the Cardiovascular and Metabolic Diseases Etiology Research Center cohort, of community-dwelling adults aged 30 to 64 without overt cardiovascular diseases, we presented distributions of tumor necrosis factor (TNF)-α and -β, interleukin (IL)-1α, -1β, and 6, monocyte chemoattractant protein (MCP)-1 and -3 and high sensitivity C-reactive protein (hsCRP) with and without non-detectable (ND) measurements using multiplex enzyme-linked immunosorbent assay. Then, we compared each markers by sex, age, and prevalence of type 2 diabetes mellitus, hypertension, and dyslipidemia, using the Wilcoxon Rank-Sum Test.
Results In general, there were inconsistencies in direction and magnitude of differences in distributions by sex, age, and prevalence of cardiometabolic disorders. Overall, the median and the 99th percentiles were higher in men than in women. Older participants had higher TNF-α, high sensitivity IL-6 (hsIL-6), MCP-1, hsCRP, TNF-β, and MCP-3 median, after excluding the NDs. Participants with type 2 diabetes mellitus had higher median for all assayed biomarkers, except for TNF-β, IL-1α, and MCP-3, in which the medians for both groups were 0.00 due to predominant NDs. Compared to normotensive group, participants with hypertension had higher TNF-α, hsIL-6, MCP-1, and hsCRP median. When stratifying by dyslipidemia prevalence, the comparison varied significantly depending on the treatment of NDs.
Conclusion Our findings provide sex-, age-, and disease-specific reference values to improve risk prediction and diagnostic performance for inflammatory diseases in both population- and clinic-based settings.
-
Citations
Citations to this article as recorded by- Characterizing CD8+ TEMRA Cells in CP/CPPS Patients: Insights from Targeted Single-Cell Transcriptomic and Functional Investigations
Fei Zhang, Qintao Ge, Jialin Meng, Jia Chen, Chaozhao Liang, Meng Zhang
ImmunoTargets and Therapy.2024; Volume 13: 111. CrossRef - Association between physical activity and inflammatory markers in community-dwelling, middle-aged adults
So Mi Jemma Cho, Hokyou Lee, Jee-Seon Shim, Justin Y. Jeon, Hyeon Chang Kim
Applied Physiology, Nutrition, and Metabolism.2021; 46(7): 828. CrossRef - The monocyte-to-lymphocyte ratio: Sex-specific differences in the tuberculosis disease spectrum, diagnostic indices and defining normal ranges
Thomas S. Buttle, Claire Y. Hummerstone, Thippeswamy Billahalli, Richard J. B. Ward, Korina E. Barnes, Natalie J. Marshall, Viktoria C. Spong, Graham H. Bothamley, Selvakumar Subbian
PLOS ONE.2021; 16(8): e0247745. CrossRef
- Characterizing CD8+ TEMRA Cells in CP/CPPS Patients: Insights from Targeted Single-Cell Transcriptomic and Functional Investigations
- Metabolic Risk/Epidemiology
- Association between the Thigh Muscle and Insulin Resistance According to Body Mass Index in Middle-Aged Korean Adults
- Ji Eun Heo, Jee-Seon Shim, Hokyou Lee, Hyeon Chang Kim
- Diabetes Metab J. 2020;44(3):446-457. Published online April 16, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0110
- 6,736 View
- 89 Download
- 8 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background We examined the associations between thigh muscle area (TMA) and insulin resistance (IR) according to body mass index (BMI) in middle-aged Korean general population.
Methods TMA was measured using quantitative computed tomography and corrected by body weight (TMA/Wt) in 1,263 men, 788 premenopausal women, and 1,476 postmenopausal women all aged 30 to 64 years. The tertiles of TMA/Wt were calculated separately for men and for premenopausal and postmenopausal women. Homeostatic model assessment for insulin resistance (HOMA-IR) was performed using fasting blood glucose and insulin levels, and increased IR was defined according to sex-specific, top quartiles of HOMA-IR. Associations between the TMA/Wt tertiles and increased IR according to the BMI categories (<25 and ≥25 kg/m2) were assessed using multivariable logistic regression analysis.
Results In men with higher BMIs, but not in those with lower BMIs, the presence of an increased IR had significantly higher odds ratios in the lower TMA/Wt tertiles, even after adjustment for visceral fat area. However, in premenopausal and postmenopausal women, there was no significant inverse association between TMA/Wt tertiles and increased IR, regardless of BMI category.
Conclusion Our findings suggest that the thigh muscle is inversely associated with IR in men, particularly in those with higher BMIs.
-
Citations
Citations to this article as recorded by- Risk of sleep apnea associated with higher blood pressure among Chinese and Korean Americans
Brittany N. Morey, Yuxi Shi, Soomin Ryu, Susan Redline, Ichiro Kawachi, Hye Won Park, Sunmin Lee
Ethnicity & Health.2024; 29(3): 295. CrossRef - Sex-specific equations to estimate body composition: Derivation and validation of diagnostic prediction models using UK Biobank
Yueqi Lu, Ying Shan, Liang Dai, Xiaosen Jiang, Congying Song, Bangwei Chen, Jingwen Zhang, Jing Li, Yue Zhang, Junjie Xu, Tao Li, Zuying Xiong, Yong Bai, Xiaoyan Huang
Clinical Nutrition.2023; 42(4): 511. CrossRef - Gender Differences in Relation to Body Composition, Insulin Resistance, and Islet Beta Cell Function in Newly Diagnosed Diabetic or Pre-Diabetic Patients
Minglei Ma, Tao Jiang, Zhen Wen, Dongxue Zhang, Lei Xiu
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 723. CrossRef - Non-Alcoholic Fatty Liver Disease with Sarcopenia and Carotid Plaque Progression Risk in Patients with Type 2 Diabetes Mellitus
Yongin Cho, Hye-Sun Park, Byung Wook Huh, Yong-ho Lee, Seong Ha Seo, Da Hea Seo, Seong Hee Ahn, Seongbin Hong, So Hun Kim
Diabetes & Metabolism Journal.2023; 47(2): 232. CrossRef - Prospective External Validation of an Algorithm Predicting Hourly
Basal Insulin Infusion Rates from Characteristics of Patients with Type 1
Diabetes Treated with Insulin Pumps
Jana S. Schmelzer, Melanie Kahle-Stephan, Juris J. Meier, Michael A. Nauck
Experimental and Clinical Endocrinology & Diabetes.2023; 131(10): 539. CrossRef - Establishing reference values for percentage of appendicular skeletal muscle mass and their association with metabolic syndrome in Korean adolescents
Da Hye Lee, Sung-Chan Kang, Seung-Sik Hwang, Yun Jeong Lee, Hwa Young Kim, Seong Yong Lee, Choong Ho Shin, Jaehyun Kim
Annals of Pediatric Endocrinology & Metabolism.2023; 28(4): 237. CrossRef - Evaluating Triglyceride and Glucose Index as a Simple and Easy-to-Calculate Marker for All-Cause and Cardiovascular Mortality
Kyung-Soo Kim, Sangmo Hong, You-Cheol Hwang, Hong-Yup Ahn, Cheol-Young Park
Journal of General Internal Medicine.2022; 37(16): 4153. CrossRef - Association between Lower-to-Upper Ratio of Appendicular Skeletal Muscle and Metabolic Syndrome
Hyun Eui Moon, Tae Sic Lee, Tae-Ha Chung
Journal of Clinical Medicine.2022; 11(21): 6309. CrossRef
- Risk of sleep apnea associated with higher blood pressure among Chinese and Korean Americans
- Metabolic Risk/Epidemiology
- Association between Non-Alcoholic Steatohepatitis and Left Ventricular Diastolic Dysfunction in Type 2 Diabetes Mellitus
- Hokyou Lee, Gyuri Kim, Young Ju Choi, Byung Wook Huh, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Eun Jig Lee, Yong-ho Lee, Kap Bum Huh
- Diabetes Metab J. 2020;44(2):267-276. Published online February 28, 2019
- DOI: https://doi.org/10.4093/dmj.2019.0001
- 6,868 View
- 150 Download
- 25 Web of Science
- 27 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Impaired diastolic heart function has been observed in persons with non-alcoholic fatty liver disease (NAFLD) and/or with type 2 diabetes mellitus (T2DM). However, it is unclear whether NAFLD fibrotic progression, i.e., non-alcoholic steatohepatitis, poses an independent risk for diastolic dysfunction in T2DM. We investigated the association between liver fibrosis and left ventricular (LV) diastolic dysfunction in T2DM.
Methods We analyzed 606 patients with T2DM, aged ≥50 years, who had undergone liver ultrasonography and pulsed-wave Doppler echocardiography. Insulin sensitivity was measured by short insulin tolerance test. Presence of NAFLD and/or advanced liver fibrosis was determined by abdominal ultrasonography and NAFLD fibrosis score (NFS). LV diastolic dysfunction was defined according to transmitral peak early to late ventricular filling (E/A) ratio and deceleration time, using echocardiography.
Results LV diastolic dysfunction was significantly more prevalent in the NAFLD versus non-NAFLD group (59.7% vs. 49.0%,
P =0.011). When NAFLD was stratified by NFS, subjects with advanced liver fibrosis exhibited a higher prevalence of diastolic dysfunction (49.0%, 50.7%, 61.8%; none, simple steatosis, advanced fibrosis, respectively;P for trend=0.003). In multivariable logistic regression, liver fibrosis was independently associated with diastolic dysfunction (odds ratio [OR], 1.58; 95% confidence interval [CI], 1.07 to 2.34;P =0.022) after adjusting for insulin resistance and cardiometabolic risk factors. This association remained significant in patients without insulin resistance (OR, 4.32; 95% CI, 1.73 to 11.51;P =0.002).Conclusions Liver fibrosis was associated with LV diastolic dysfunction in patients with T2DM and may be an independent risk factor for diastolic dysfunction, especially in patients without systemic insulin resistance.
-
Citations
Citations to this article as recorded by- Anti-hepatopathy and anti-nephropathy activities of Taraxacum officinale in a rat model of Streptozotocin diabetes-induced hepatorenal toxicity and dyslipidemia via attenuation of oxidative stress, inflammation, apoptosis, electrolyte imbalances, and mito
Sunday Aderemi Adelakun, Aniah Julius Akomaye, Olusegun Dare Omotoso, Olukayode Abimbola Arowosegbe
Aspects of Molecular Medicine.2024; 3: 100034. CrossRef - Epidemiology of heart failure in diabetes: a disease in disguise
Anna G. Hoek, Elisa Dal Canto, Eva Wenker, Navin Bindraban, M. Louis Handoko, Petra J. M. Elders, Joline W. J. Beulens
Diabetologia.2024; 67(4): 574. CrossRef - NASH triggers cardiometabolic HFpEF in aging mice
Dániel Kucsera, Mihály Ruppert, Nabil V. Sayour, Viktória E. Tóth, Tamás Kovács, Zsombor I. Hegedűs, Zsófia Onódi, Alexandra Fábián, Attila Kovács, Tamás Radovits, Béla Merkely, Pál Pacher, Péter Ferdinandy, Zoltán V. Varga
GeroScience.2024;[Epub] CrossRef - Inhibition of visceral adipose tissue-derived pathogenic signals by activation of adenosine A2AR improves hepatic and cardiac dysfunction of NASH mice
Chia-Chang Huang, Hsiao-Yun Yeh, Roger Lin, Tsai-Ling Liao, Hsiao-Chin Shen, Ying-Ying Yang, Han-Chieh Lin
American Journal of Physiology-Gastrointestinal and Liver Physiology.2024; 326(4): G385. CrossRef - Is non-alcoholic fatty liver disease a sign of left ventricular diastolic dysfunction in patients with type 2 diabetes mellitus? A systematic review and meta-analysis
Sicheng Wang, Xiangyuan Zhang, Qiqi Zhang, Boxun Zhang, Linhua Zhao
BMJ Open Diabetes Research & Care.2023; 11(1): e003198. CrossRef - The effect of metabolic dysfunction-associated fatty liver disease and diabetic kidney disease on the risk of hospitalization of heart failure in type 2 diabetes: a retrospective cohort study
Seung Eun Lee, Juhwan Yoo, Bong-Seong Kim, Han Seok Choi, Kyungdo Han, Kyoung-Ah Kim
Diabetology & Metabolic Syndrome.2023;[Epub] CrossRef - Metabolic Dysfunction-Associated Fatty Liver Disease and Mortality: A Population-Based Cohort Study
Kyung-Soo Kim, Sangmo Hong, Hong-Yup Ahn, Cheol-Young Park
Diabetes & Metabolism Journal.2023; 47(2): 220. CrossRef - Therapies for patients with coexisting heart failure with reduced ejection fraction and non-alcoholic fatty liver disease
Jose Arriola-Montenegro, Renato Beas, Renato Cerna-Viacava, Andres Chaponan-Lavalle, Karla Hernandez Randich, Diego Chambergo-Michilot, Herson Flores Sanga, Pornthira Mutirangura
World Journal of Cardiology.2023; 15(7): 328. CrossRef - Non-alcoholic Fatty Liver Disease and Its Association With Left Ventricular Diastolic Dysfunction: A Systematic Review
Namra V Gohil, Nida Tanveer, Vijaya Krishna Makkena, Arturo P Jaramillo, Babatope L Awosusi, Javaria Ayyub, Karan Nareshbhai Dabhi, Tuheen Sankar Nath
Cureus.2023;[Epub] CrossRef - Associations of advanced liver fibrosis with heart failure with preserved ejection fraction in type 2 diabetic patients according to obesity and metabolic goal achievement status
Wangyan Jiang, Zhelong Liu, Shaohua Liu, Tingting Du
Frontiers in Endocrinology.2023;[Epub] CrossRef - Non-Alcoholic Fatty Liver Disease and Echocardiographic Parameters of Left Ventricular Diastolic Function: A Systematic Review and Meta-Analysis
Athina Goliopoulou, Panagiotis Theofilis, Evangelos Oikonomou, Artemis Anastasiou, Panteleimon Pantelidis, Maria Ioanna Gounaridi, Georgios E. Zakynthinos, Ourania Katsarou, Eva Kassi, Vaia Lambadiari, Dimitris Tousoulis, Manolis Vavuranakis, Gerasimos Si
International Journal of Molecular Sciences.2023; 24(18): 14292. CrossRef - Non-alcoholic fatty liver disease and risk of cardiovascular diseases: clinical association, pathophysiological mechanisms, and management
Rong Yang, Jian-Gao Fan
Cardiology Plus.2023; 8(4): 217. CrossRef - Association of cardiovascular factors in diabetic patients with non-alcoholic fatty liver disease
Evangelos Cholongitas, Dimitrios Tsilingiris, Panagiota Diamantopoulou, Elpida Mastrogianni, Anastasios Tentolouris, Dimitrios Karagiannakis, Ioannis Moyssakis, George V. Papatheodoridis, Nikolaos Tentolouris
Hormones.2022; 21(1): 133. CrossRef - Non-alcoholic fatty liver disease association with structural heart, systolic and diastolic dysfunction: a meta-analysis
Jie Ning Yong, Cheng Han Ng, Chloe Wen-Min Lee, Yu Yi Chan, Ansel Shao Pin Tang, Margaret Teng, Darren Jun Hao Tan, Wen Hui Lim, Jingxuan Quek, Jieling Xiao, Yip Han Chin, Roger Foo, Mark Chan, Weiqin Lin, Mazen Noureddin, Mohammad Shadab Siddiqui, Mark D
Hepatology International.2022; 16(2): 269. CrossRef - Triglyceride and glucose index is a simple and easy‐to‐calculate marker associated with nonalcoholic fatty liver disease
Kyung‐Soo Kim, Sangmo Hong, Hong‐Yup Ahn, Cheol‐Young Park
Obesity.2022; 30(6): 1279. CrossRef - Association of Metabolic Dysfunction-Associated Fatty Liver Disease With Left Ventricular Diastolic Function and Cardiac Morphology
Dandan Peng, Zhenqiu Yu, Mingwei Wang, Junping Shi, Lei Sun, Yuanyuan Zhang, Wenbin Zhao, Chen Chen, Jiake Tang, Chunyi Wang, Jie Ni, Wen Wen, Jingjie Jiang
Frontiers in Endocrinology.2022;[Epub] CrossRef - NAFLD in Cardiovascular Diseases: A Contributor or Comorbidity?
Bing Chen, W.H. Wilson Tang, Mario Rodriguez, Kathleen E. Corey, Arun J. Sanyal, Patrick S. Kamath, Biykem Bozkurt, Hafeez Ul Hassan Virk, Gregg S. Pressman, Jeffrey V. Lazarus, Hashem B. El-Serag, Chayakrit Krittanawong
Seminars in Liver Disease.2022; 42(04): 465. CrossRef - Nonalcoholic fatty liver disease is associated with early left ventricular diastolic dysfunction in patients with type 2 diabeteS
Walaa Sheba, Eman Morsy, Salah Altahan, Mona Ayaad, Sameh A. Lashen
Alexandria Journal of Medicine.2022; 58(1): 117. CrossRef - Cardiac abnormalities in patients with nonalcoholic fatty liver disease
Yu Dong, Guangsen Li
Herz.2021; 46(2): 158. CrossRef - Elafibranor improves diet-induced nonalcoholic steatohepatitis associated with heart failure with preserved ejection fraction in Golden Syrian hamsters
François Briand, Julie Maupoint, Emmanuel Brousseau, Natalia Breyner, Mélanie Bouchet, Clément Costard, Thierry Leste-Lasserre, Mathieu Petitjean, Li Chen, Audrey Chabrat, Virgile Richard, Rémy Burcelin, Caroline Dubroca, Thierry Sulpice
Metabolism.2021; 117: 154707. CrossRef - Association of the Non‐Alcoholic Fatty Liver Disease Fibrosis Score with subclinical myocardial remodeling in patients with type 2 diabetes: A cross‐sectional study in China
Nengguang Fan, Xiaoying Ding, Qin Zhen, Liping Gu, Aifang Zhang, Tingting Shen, Yufan Wang, Yongde Peng
Journal of Diabetes Investigation.2021; 12(6): 1035. CrossRef - Nonalcoholic fatty liver disease, diastolic dysfunction, and impaired myocardial glucose uptake in patients with type 2 diabetes
Minyoung Lee, Kwang Joon Kim, Tae‐Ha Chung, Jaehyun Bae, Yong‐ho Lee, Byung‐Wan Lee, Bong‐Soo Cha, Mijin Yun, Eun Seok Kang
Diabetes, Obesity and Metabolism.2021; 23(4): 1041. CrossRef - Interplay between Heart Disease and Metabolic Steatosis: A Contemporary Perspective
Mohammad Said Ramadan, Vincenzo Russo, Gerardo Nigro, Emanuele Durante-Mangoni, Rosa Zampino
Journal of Clinical Medicine.2021; 10(8): 1569. CrossRef - Correlation Between 25-Hydroxyvitamin D Level and Cardiac Diastolic Dysfunction in Chinese Adults with Early-Onset Type 2 Diabetes Mellitus: A Cross-Sectional Study
Lei Xiu, Xiao-ai Yao, Tao Jiang
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2021; Volume 14: 1823. CrossRef - Bi-directional and temporal relationship between elevated alanine aminotransferase and hypertension in a longitudinal study of Chinese adults
Guoxin Huang, Hui Zhou, Chao Shen, Yihui Sheng, Ruyu Xue, Chen Dong, Shaoyan Zhang
Clinical and Experimental Hypertension.2021; 43(8): 750. CrossRef - Response: Association between Non-Alcoholic Steatohepatitis and Left Ventricular Diastolic Dysfunction in Type 2 Diabetes Mellitus (Diabetes Metab J 2020;44:267–76)
Hokyou Lee, Gyuri Kim, Yong-ho Lee
Diabetes & Metabolism Journal.2020; 44(3): 486. CrossRef - Letter: Association between Non-Alcoholic Steatohepatitis and Left Ventricular Diastolic Dysfunction in Type 2 Diabetes Mellitus (Diabetes Metab J2020;44:267–76)
Sung Hoon Yu
Diabetes & Metabolism Journal.2020; 44(3): 482. CrossRef
- Anti-hepatopathy and anti-nephropathy activities of Taraxacum officinale in a rat model of Streptozotocin diabetes-induced hepatorenal toxicity and dyslipidemia via attenuation of oxidative stress, inflammation, apoptosis, electrolyte imbalances, and mito

 KDA
KDA
 First
First Prev
Prev





